

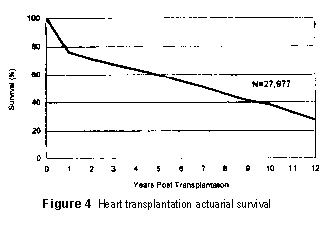
 Figure 4 shows the actuarial survival after heart transplantation
over a 12-year period. The overall 1-year survival for heart transplantation is 76%,
and thereafter there is approximately a 4% mortality per year over the subsequent
11 years. This fall off in survival is almost a straight line from year 1 through
year 12 and projected out would represent a maximum survival of approximately 18
1/2 years for the entire population.
Figure 4 shows the actuarial survival after heart transplantation
over a 12-year period. The overall 1-year survival for heart transplantation is 76%,
and thereafter there is approximately a 4% mortality per year over the subsequent
11 years. This fall off in survival is almost a straight line from year 1 through
year 12 and projected out would represent a maximum survival of approximately 18
1/2 years for the entire population.
As has been previously shown in prior Registry reports, risk factors that has statistically significant impact on 1-year mortality include prior transplantation, requiring ventricular assistance or ventilator support before transplantation, the very old and very young, recipient or donor of female gender, underlying congenital cardiac disease, centers performing low numbers (less than nine) of transplantations per year, and the older donor. In fact, a heart donor older than 60 years of age has a relative odds ratio equivalent in negative impact to repeat transplantation. The ischemic time (duration of heart shut down during transplantation procedure - Ed.) continues to be a risk factor and, analyzed as a continuous variable adds approximately a 10% increase in mortality for every hour of ischemia. Finally, for the first time, patients at risk for primary cytomegalovirus (donor positive/ recipient negative)carries a 20% chance ofincreased 1-year mortality.
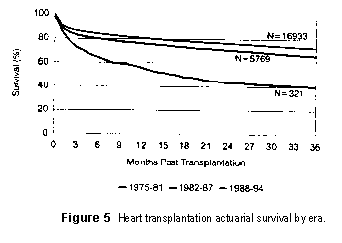 Figure 5 shows 3-year actuarial survival by era. Continuous
improvement has occurred in heart transplantation outcomes over the past 20 years
- the most recent era (1988 through 1994) with a 1-year survival rate of 81% and
a 3-year survival rate of 73%.
Figure 5 shows 3-year actuarial survival by era. Continuous
improvement has occurred in heart transplantation outcomes over the past 20 years
- the most recent era (1988 through 1994) with a 1-year survival rate of 81% and
a 3-year survival rate of 73%.
A new analysis by the Registry only includes those patients who have survived at least 12 months after heart transplantation and investigates potential risk factors for survival beyond that time. In this analysis, three factors appear to negatively affect survival in this subgroup of patients: those patients who had undergone retransplantation, those patients who received an organ from a donor who was 55 or more years of age, and those patients whose donor died of a cerebral vascular accident. It is noteworthy that a donor age of 55 or greater carried the same risk as that of a prior transplantation even in this "survivor" population.
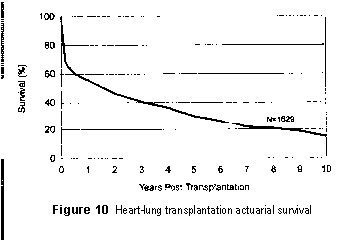 The number of heart-lung transplant procedures peaked in 1989
and has continued to decline since that time, presumably because of the increase
in the use of single and double lung transplant procedures. The three primary indications
for heart-lung transplantation are pulmonary hypertension, congenital heart disease,
and cystic fibrosis. The actuarial 10-year survival for heart-lung is shown in Figure
10. The 1-year survival is approximately 56% with the 10 year survival being less
than 20%. As shown in previous Registry reports, being on a ventilator before transplantation,
being a male recipient, and having a donor older than 40 years continue to be statistically
significant negative risk factors.
The number of heart-lung transplant procedures peaked in 1989
and has continued to decline since that time, presumably because of the increase
in the use of single and double lung transplant procedures. The three primary indications
for heart-lung transplantation are pulmonary hypertension, congenital heart disease,
and cystic fibrosis. The actuarial 10-year survival for heart-lung is shown in Figure
10. The 1-year survival is approximately 56% with the 10 year survival being less
than 20%. As shown in previous Registry reports, being on a ventilator before transplantation,
being a male recipient, and having a donor older than 40 years continue to be statistically
significant negative risk factors.
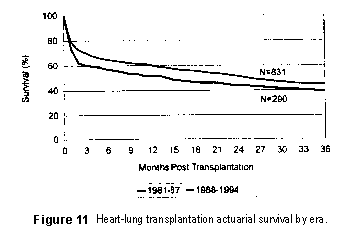 Figure 11 shows 3-year actuarial survival for two eras of heart-lung
transplantation. Survival has improved in the more current era of 1988 throu gh 1994.
Figure 11 shows 3-year actuarial survival for two eras of heart-lung
transplantation. Survival has improved in the more current era of 1988 throu gh 1994.
A number of additional risk factors for 1 year mortality after single lung transplantation have been identified since the last Registry report. In addition to the known risk factor of preoperative ventilator support, patients with congenital heart disease, prior transplantation, underlying diseases of primary pulmonary hypertension and idiopathic pulmonary fibrosis, and older age all appear to be significant risk factors for 1-year mortality. Moreover, those patients who are at primary risk for cytomegalovirus have a 1.6-fold increase in mortality in the first year.
In those patients over the age of 60 years at the time of single lung transplantation the 2-year actuarial survival rate is 51% versus 61% for those under that age. In those patients who have reached the first anniversary of their transplantations, only one factor, the underlying diagnosis of interstitial pulmonary Fibrosis, negatively affected long-term survival beyond 1 year.
The 30-day mortality is consistently the highest for the less than 1 year age group. The survival for heart transplantation for patients 6 through 18 years of age is similar to that seen for the adult population, with a demonstrated increased mortality for the younger age groups. Most of this increase in mortality appears to be in that first 30-day period (operative mortality).
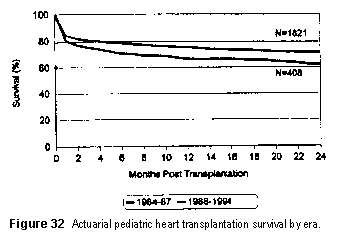 Figure 32 shows the actuarial survival for pediatric heart transplantation
by era. There has been some improvement in 1- and 2-year survival in the most recent
transplant era (1988 through 1994, and again the difference in survival appears to
be predominately due to differences in early post operative survival (6 months).
Figure 32 shows the actuarial survival for pediatric heart transplantation
by era. There has been some improvement in 1- and 2-year survival in the most recent
transplant era (1988 through 1994, and again the difference in survival appears to
be predominately due to differences in early post operative survival (6 months).
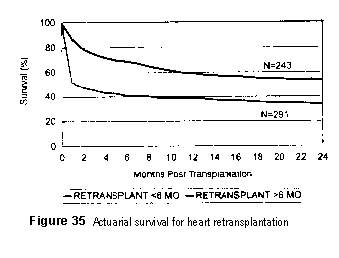
In heart retransplantation in the pediatric population, the 12- and 24-month survivals are 47% and 40%, respectively. These results are not as favorable as those for adult heart retransplantation performed more than 6 months after original transplantation; however, the results are somewhat better than those for early retransplantation for adults. The results of heart-lung and lung retransplantation in the pediatric age population are comparable with those seen in the adult population.
Despite continued improvement in outcomes in the modern era of thoracic transplantation, survival is far from ideal. We will continue to perform analyses of risk factors for long-term outcome with the goal of assisting transplant physicians and surgeons in issues of patient selection and organ allocation.


 How about that guy who shot himself so his organs could be donated.
This is true. Trouble was no one believed him for one thing, for another he put the
gun to the center of his chest, by the time they found him nothing was usable, except
his brain, and it was rejected as not being very good even when it was hooked up
and running.
How about that guy who shot himself so his organs could be donated.
This is true. Trouble was no one believed him for one thing, for another he put the
gun to the center of his chest, by the time they found him nothing was usable, except
his brain, and it was rejected as not being very good even when it was hooked up
and running.
There is a gentleman heart "plant" out there who has received the Heart Laser treatment discussed in the October UpBeat. He feels he may be the only Tx to have received the treatment of piercing 15 to 30 one millimeter holes through the outside wall on the left side of the heart to create a secondary circulation system. Says he feels "like a new man." His ejection fraction due to TCAD was down into the 20's, but after the procedure he's back up into the high 40's and holding. Hopefully, he'll come through with a story on this great potential for Tx's on the decline.
Once again, please remember our adage, "If you can read the annual Transplantation Registry report, you're way ahead of the game."
Remember, nothing keeps the cholesterol levels down like leaving it on the table or in the refrigerator. As usual after 7 11/12th years now, there's no December issue of UpBeat, mainly to just "give it a rest." And thank you all for the donations, large and small, they will make it possible to launch forth into the 8th full year of UpBeat.
The telltale chemical, known as tissue plasminogen activator or t-PA, is made by the muscle cells lining the blood vessels of the heart. The researchers said it seemed to have the ability to prevent clogging in arteries feeding the heart muscle, a problem that often faces transplant patients.
In tests of 78 patients over five years, a team led by Dr. Carlos Labarrere of Methodist Hospital in Indianapolis, Indiana found that if the transplanted heart kept producing t-PA, the heart was far more likely to survive and less likely to develop new heart disease than a heart whose t-PA producing capacity declined.
Among the 40 volunteers whose new hearts started making less t-PA soon after the transplant, the heart arteries of 78 percent began to clog up within three months. Among the hearts with normal t-PA levels, heart disease developed in only 24 percent of cases.
While only one person with normal t-PA levels died or needed a second transplant, 12 t-PA-deficient volunteers fell into that category, the researchers found. Overall, when the heart stopped making t-PA, a patient was eight times more likely to develop heart disease in the new heart and 25 times more likely to have the heart fail.
Why a transplanted heart would stop making the normal amount of t-PA is not known. But because doctors could detect a decline in the t-PA level two or three weeks after a transplant, "we can conservatively conclude that the depletion of t-PA is an early sign of the development of coronary artery disease," the researchers said.
DENVER (AP 10/18/95) --A substance found in rodent testicles may hold the key to preventing the rejection of transplanted organs, researchers said Wednesday.
A research team at the University of Colorado Health Sciences Center found that a molecule called CD95 ligand in the testicles of mice appears to prevent the rodents' immune systems from killing transplanted cells.
'This could be the magic elixir that we can use to prevent graft rejection," said Donald Bellgrau, associate professor of 'immunology who reported the results Wednesday in the journal Nature.
The human body's immune system is the greatest enemy of transplanted cells and organs. But scientists have known that the testicles, brain and eyes are "privileged sites" where immunosuppression doesn't work. Bellgrau's team set out to find why.
They learned that Sertoli cells in mice testicles emit CD95 ligand, which appears responsible for the immunosuppressant effect, and were able to isolate CD95 molecules. Then, they found that grafts of testes from different mice weren't rejected, as long as they had contained the CD95 molecules.
The researchers are a long way from testing the molecule in humans.
Also, another scientist cautioned in an accompanying editorial that while CD95 ligand may be a necessary factor, more research must be done to show that it can prevent immunosuppression by itself. Other, unknown factors could come into play, wrote David L. Vaux of the Walter and Eliza Hall Institute of Medical Research in Victoria, Australia.
Bellgrau said the next step is to implant the CD95 gene in another cell to find out if it becomes a "privileged site," resistant to graft rejection just like the Sertoli cells inside the mice testicles.
"We have two basic protocols we want to test: one as a drug; then putting it in cells and making it resistant to graft rejections," Bellgrau said.
Vaux wrote that doubts about CD95 ligand won't be settled until transplant experiments can be performed on mice that are genetically engineered to contain the molecule throughout their body.
Eventually, Bellgrau hopes CD95 ligand could be used to prevent rejection of islet cells transplanted for treatment of Type I diabetes, and improve the success rate of organ transplants in general.
Bellgrau's team also included Dr. Alex Franzusoff, associate professor of cellular and structural biology; Richard Duke, assistant professor of medicine; and Jodene Moore, an immunology graduate student.
Dr. O.H. Frazier, director of surgical research and chief of cardiac transplantation at the Texas Heart Institute, is a co-principal investigator. He and Jarvik, the principal investigator, have been brainstorming the idea for a decade.
"Our goal is to develop this as an alternative to transplant," he said. "But time will only tell if we are successful."
Frazier, terming the device as "simple technology," said it requires no valves and minimal energy input.
The small pump will be placed directly in the left ventricle, the main pumping chamber of the heart.
The pump will be developed under a five-year $5.8 million federal contract in conjunction with Transicoil Inc. of Valley Forge, Pa., and Bell Communications Research of Red Bank, N.J. Jarvik' s primary affiliation is with Transicoil, said heart institute spokesman Mark Mattson.
Officials estimated that 50,000 people each year could benefit from a heart transplant, but only 2,000 receive them. If this pump proves effective, it could help them as well 3 million who are treated for congestive heart failure annually, Frazier said.
Heart failure, which occurs when the organ loses its mechanical ability to pump blood effectively, can be caused by a variety of factors.
Abel Goodman, 64, underwent the privately funded operation last Monday at the John Radcliffe Hospital in Oxford, with the help of experts from the Texas Heart Institute.
The artificial heart, which is powered by external batteries through a lead in Goodman's skin, was stitched to the left ventricle of his natural heart. It pumps oxygenated blood around the body.
"Although there is always a danger of infection and blood clots with these devices, we believe we have minimized the risk," Tim Myers, research coordinator of the Texas Hearth Institute, told the newspaper. "We hope this patient will live for years."
Goodman, who was too old to be eligible for a natural heart transplant on the National Health Service and was told he had six months to live, was selected from 25 volunteers for the surgery.
He suffered a mild stroke on Saturday but cardiac surgeon Steve Westaby gave him a 90 percent chance of full recovery.
Goodman will have to recharge the 2 1/2 pound (1.13 kg) batteries, which are strapped to his waist, every eight hours for the rest of his life.
Myers explained that the operation was carried out in Britain because of problems with the U.S. Food and Drug Administration, which would only approve the electronic heart as a bridge to getting a human heart within two years,
The electric heart, which cost 65,000 pounds ($102,050) to develop and was manufactured by Thermo Cardiosystems Inc. (TCI) of Boston, is thought to have a lower chance of rejection and infection compared to animal hearts.
More than nine percent of women patients receiving a liver from a male donor developed chronic rejection, compared with 1.7 percent of those given another woman's liver, the doctors reported in the Lancet medical journal.
The reason may be that the female recipient has an "immune attack" against the maleness of the transplanted organ, they said.
Chronic rejection usually means the new liver has to be removed.
The team from Birmingham's Queen Elizabeth Hospital found that patients under the age of 30 were also more likely to develop chronic rejection, because they have more robust immune systems which reject the alien organ.
The discoveries are important because isolating risk factors could help to reduce the rate of chronic transplant rejection by more precise selection of organs for donation.
BECOMING PART PIG: Many people would be willing to accept an organ donation from a pig or other animal, according to a survey by the National Kidney Foundation in New York. Surveyors found that people worried about how little doctors know about transplanting organs between species and about the possibility of getting an animal disease from the transplant. However, every day eight or nine people die while waiting for a suitable organ. The shortage of human organs convinced 74 percent of the 2,000 people surveyed that an organ from another animal would be acceptable.
Several studies have linked transplant recipients with a higher cancer rate. Some doctors say this could be due to drugs that suppress the body's immune system given to transplant recipients to prevent their bodies from rejecting the donor organs.
Two letters to the Lancet medical journal described the problem.
Jane McGregor and Charlotte Proby of St Thomas's Hospital in London said 27 percent of more than 500 transplant patients developed skin cancer over 25 years.
They said doctors should be on the lookout for cancers in such patients.
"They often took clinically innocuous and are easy to miss,"' they wrote. "Moreover; they are frequently multiple and may grow rapidly, sometimes requiring extensive and repeated surgery for adequate control."

Internet: donmarsh@inna.net
Don Marshall
P.O. Box 482
Mathews, VA 23109-0482
804-725-3686
Compuserve 74016,1725
FAX 804-725-3686
Internet: donmarsh@inna.net
Site Stats: